GRS News
Medical Device Regulations 2023 – UK Amendments
21/6/2023
On 9th June 2023, the UK parliament passed and approved 2023 No. 627 MEDICAL DEVICES The Medical Devices (Amendment) (Great Britain) Regulations 2023.
This amendment was published after a public consultation which emphasised the need to include the EU’s supply chain safety attempt of amending the EU Medical Device Regulation [MDR 2017/745] and In-Vitro Diagnostic Regulations [EU IVDR 2017/746], with REGULATION (EU) 2023/607 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL which lays down transitional provisions for certain medical devices and in vitro diagnostic medical devices.
According to the EU’s decision, certain eligible Medical Devices and In-Vitro Diagnostic Devices will follow the following timeline:
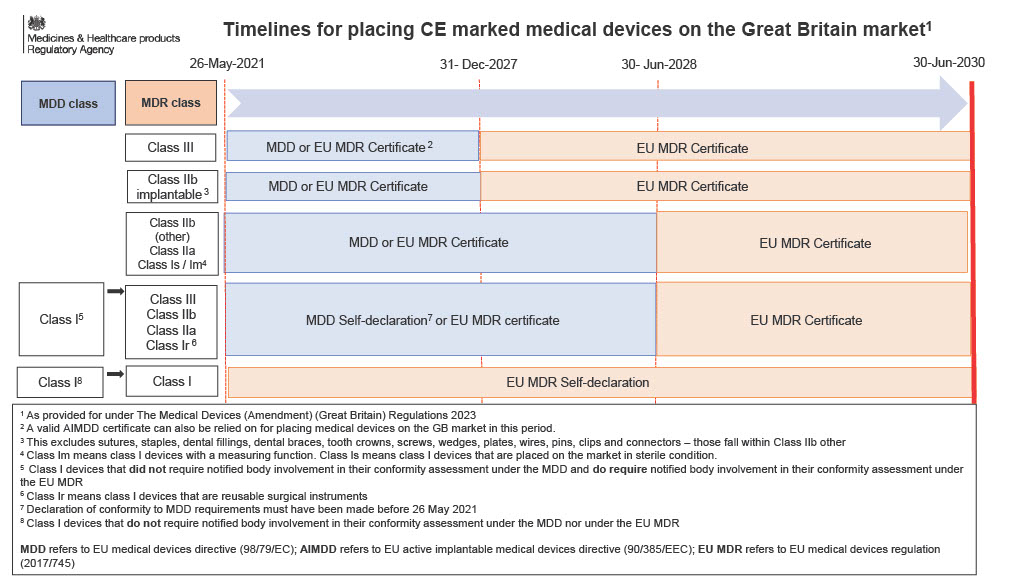
- Class III Implantable custom-made devices: 26th May 2026
- Class III & Class IIb devices: 31st December 2027
- Class IIb non implantable: 31st December 2028
- Class IIb sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips and connectors : 31st December 2028
- Class IIa and Class I (Is/Im): 31st December 2028
- Extended validity of certificates issued up to 26th May 2021
- Removes the 'sell-off' date for MDR & IVDR
These extended timelines do not change the deadline for compliance with the MDR and IVDR, which remain 26th May 2021 and 26th May 2022, respectively.
The UK government amended Medical Device Regulation (2002) to avoid disruption and create a smooth flow. This amendment will be cited as the Medical Devices (Amendment) (Great Britain) Regulations 2023. Medical device manufacturers must be reminded that the new amendments published on 9th June 2023 apply to Great Britain [ England, Scotland and Wales ]. Northern Ireland continues to follow EU Regulatory requirements with an obligatory requirement to adapt the Medical Devices (Amendment) (Great Britain) Regulations.
What does the recent publication from the UK look like?
Manufacturers and industry members enthusiastically welcomed this amendment as it gave them more time to adjust. While the industry may be rejoicing at the news of the amended timeline, regulatory professionals are warning manufacturers to use this time wisely and make sure that they are fully compliant before the deadline expires.
Important notes from the publication:
- These Regulations amend the Medical Devices Regulations 2002 to extend the periods for which certain medical devices that comply with EU legislation can be placed on the market in Great Britain.
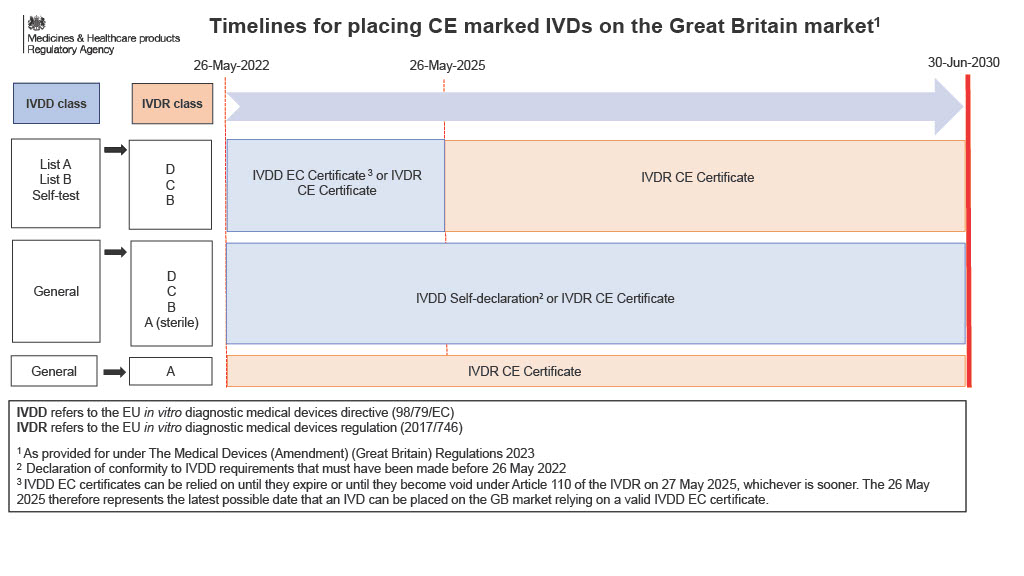
- The new regulation 1ZA sets out different dates ranging from 30th June 2023 to 30th June 2030. The effect of these new dates is that the period for placing devices on the market in Great Britain under regulations 19B and 30A (except custom-made devices), and 19C, 44ZA and 44ZB is extended.
- The manufacturer must ensure the certificate of conformity for the relevant device is valid before placing the device on the market.
- The amendments to regulations 19B, 30A and 44ZA provide that certificates issued under Directive 90/385/EEC, Directive 93/42/EEC or Directive 98/79/EC that remain valid by virtue of the transitional provisions in Regulation (EU) 2017/745 or Regulation (EU) 2017/746, are considered valid for placing devices on the market in accordance with regulations 19B, 30A and 44ZA.
- Details of the extension can be seen in the image below.
Irrespective of these amendments, manufacturers need to
- Make sure your Quality Management Systems are in Place.
- Ensure any Class 1 legacy devices which are not up-classified by the EU MDR are in compliance with the EU MDR or UK MDR 2002 (as amended) before placing on the UK market.
- Ensure appropriate transition plans from EU Medical Device Directives [EU MDD] to EU Medical Device Regulation in accordance with UK Medical Device Regulation are in place.
- Contact experts at GRS who can support you with completing your transition plans, technical files and documents.
- If you are yet to launch your medical device on the EU or UK Market, speak to our experts, who can create a comprehensive Regulatory Roadmap for a smooth launch.
Our experts are driving innovation to market success™
Drop us an email at grs@globalregulatoryservices.com or fill out an interest form https://mailchi.mp/4581bff533d0/ukmdr






